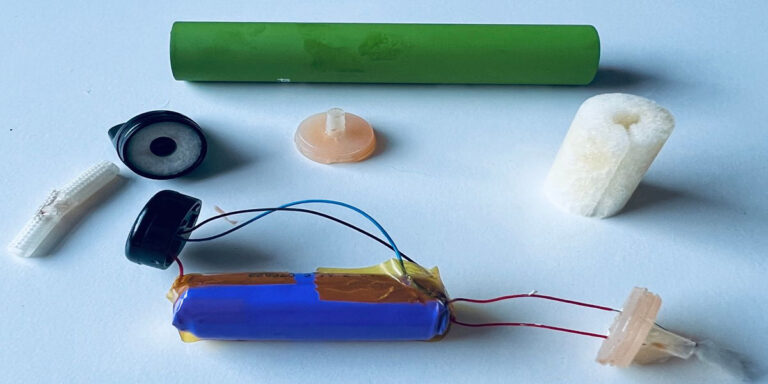
The groundbreaking launch of the world’s first clinically approved nicotine inhaler by Ayrton Saunders Limited, designed to help smokers quit smoking effectively.
On June 25, 2024, Ayrton Saunders Limited, a UK-based pharmaceutical company, announced the approval of its next-generation nicotine inhalation system by the UK Medicines and Healthcare products Regulatory Agency (MHRA). This milestone marks the introduction of the world’s first and only clinically approved nicotine inhaler, designed to aid smokers in substituting, reducing, and ultimately quitting smoking. This innovative product represents a significant advancement in Nicotine Replacement Therapy (NRT).
Product Details
MHRA Approval
The newly approved product is the first NRT inhaler to receive clinical approval from the MHRA. This endorsement validates the inhaler’s efficacy and safety, making it a reliable option for smokers seeking to quit.
Advanced Technology
The inhaler utilizes globally patented technology similar to that used in asthma inhalers. It delivers small amounts of nicotine rapidly through the lungs, mimicking the sensation of smoking and satisfying nicotine cravings effectively.
User Experience
Designed with the user in mind, the inhaler features a fresh taste, no noticeable odor, and no visible exhalation, enhancing the overall user experience. These features make it a discreet and pleasant alternative to traditional smoking and other NRT products.
Product Components
The inhaler consists of a reusable handheld, breath-activated device and a stable flavored nicotine solution. This design ensures ease of use and a consistent delivery of nicotine.
Operation Mechanism
Driven by a pressurized propellant, the inhaler requires no batteries or heating elements. This mechanism ensures that the aerosol generated does not alter the chemical structure of the nicotine formulation, maintaining its purity and effectiveness.
Market and Availability
General Sales List Medicine
As a General Sales List (GSL) medicine, the nicotine inhaler can be sold with or without a prescription in the UK. This flexibility allows it to be widely available through various channels.
Sales Channels
The product can be advertised and sold on major e-commerce platforms, in pharmacies, and through direct-to-consumer channels. This multi-channel availability ensures that consumers can easily access the product.
Market Potential
EU NRT Market
The European Union’s NRT market is currently valued at approximately $1 billion annually, with projections to exceed $1.5 billion within the next five years. The introduction of this clinically approved inhaler is expected to capture a significant share of this growing market.
Global Market
Globally, the NRT market is valued at over $3.8 billion. Ayrton Saunders’ innovative product has the potential to make a substantial impact worldwide, providing an effective solution for millions of smokers.
Future Plans
Partnerships and Expansion
Ayrton Saunders is actively seeking sales or licensing partners to launch the product in the UK. The company also plans to expand into key markets such as the EU, US, Japan, and the Middle East, leveraging its robust supply chain.
Production Capacity
The company has established a supply chain capable of delivering up to 18 million units annually. With appropriate investment, this capacity can be further scaled to meet increasing demand.
Development Process
Innovative Leadership
The development of the nicotine inhaler was led by Gerry O’Brien, company director and Royal Pharmaceutical Society Fellow. Over the past three years, the team has focused on developing and extensively redesigning the product to enhance its functionality and user experience while reducing consumer costs.
Comprehensive Redesign
The team’s efforts have resulted in an advanced, user-friendly device that meets the highest standards of safety and efficacy. This comprehensive redesign ensures that the product is both effective and appealing to smokers seeking to quit.
FAQs
What makes Ayrton Saunders’ nicotine inhaler unique?
The inhaler is the first and only clinically approved nicotine inhaler designed to help smokers quit smoking, utilizing advanced technology similar to asthma inhalers.
How does the nicotine inhaler work?
The inhaler uses a pressurized propellant to deliver small amounts of nicotine rapidly through the lungs without altering the chemical structure of the nicotine formulation.
Can the nicotine inhaler be purchased without a prescription?
Yes, the nicotine inhaler is classified as a General Sales List (GSL) medicine, meaning it can be sold with or without a prescription in the UK.
What is the market potential for this product?
The EU NRT market is valued at approximately $1 billion annually, with projections to exceed $1.5 billion within the next five years. The global market is valued at over $3.8 billion.
Who led the development of the nicotine inhaler?
The development was led by Gerry O’Brien, company director and Royal Pharmaceutical Society Fellow, over the past three years.
What are Ayrton Saunders’ future plans for the nicotine inhaler?
The company plans to seek sales or licensing partners for the UK launch and expand into the EU, US, Japan, and the Middle East, with a robust supply chain capable of delivering up to 18 million units